By: Suhana Sigh, Delaney Plecha, and Chirag Shah MD MPH
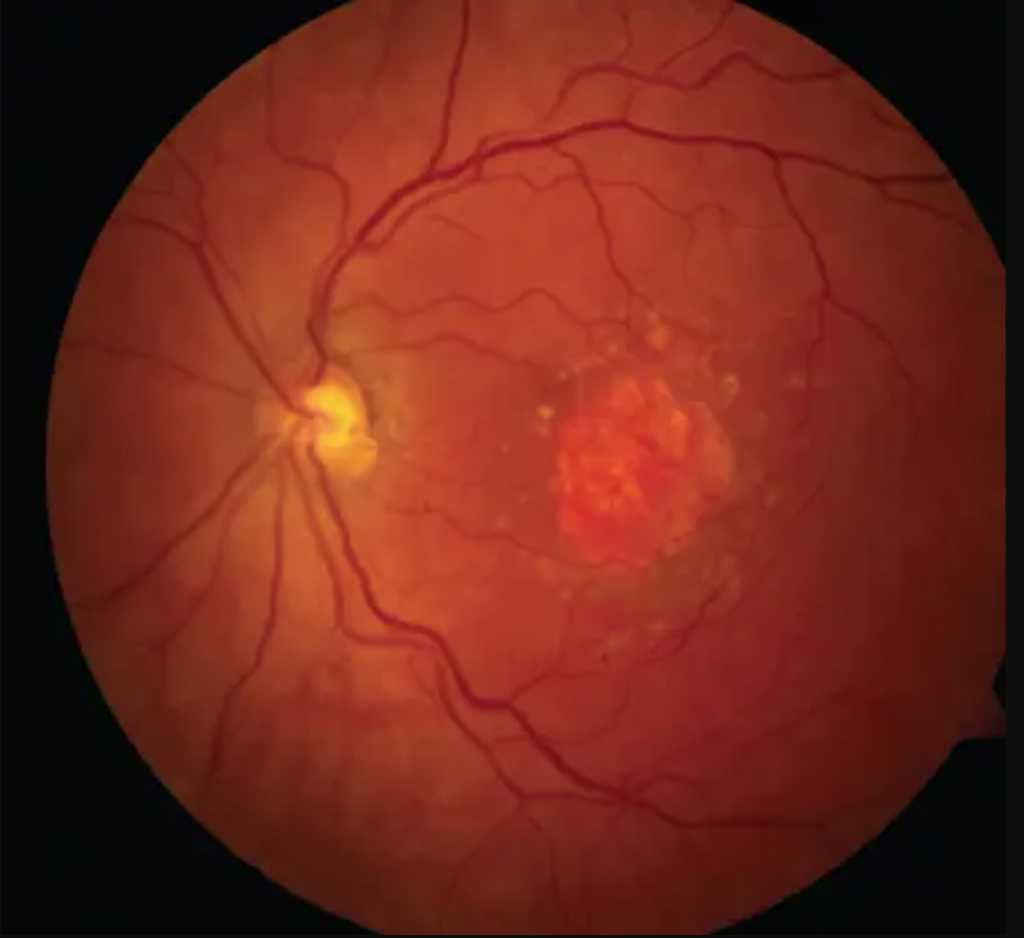
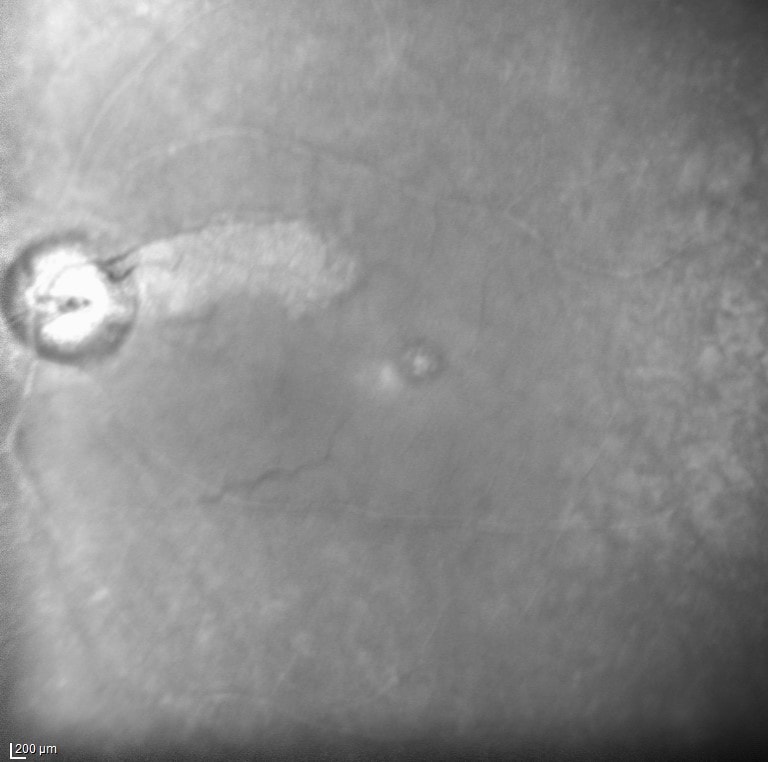
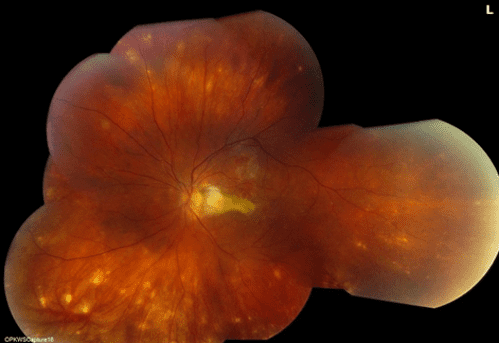
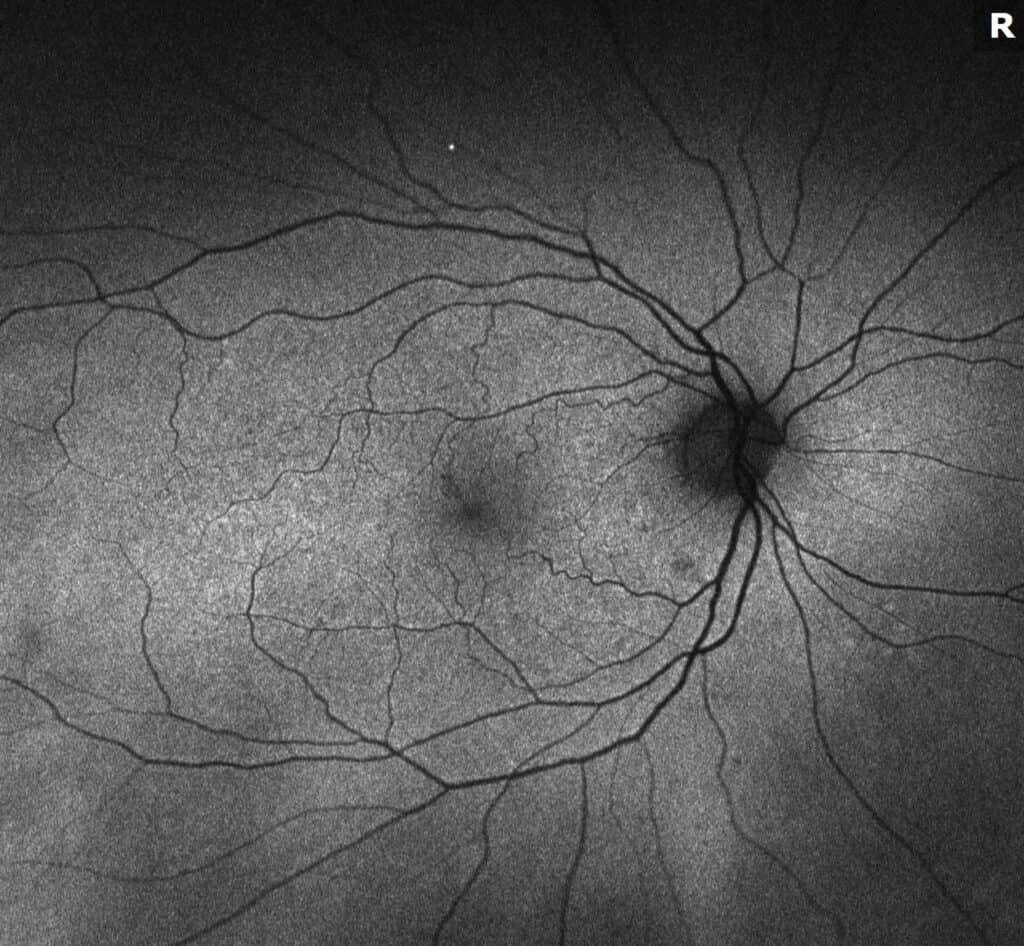
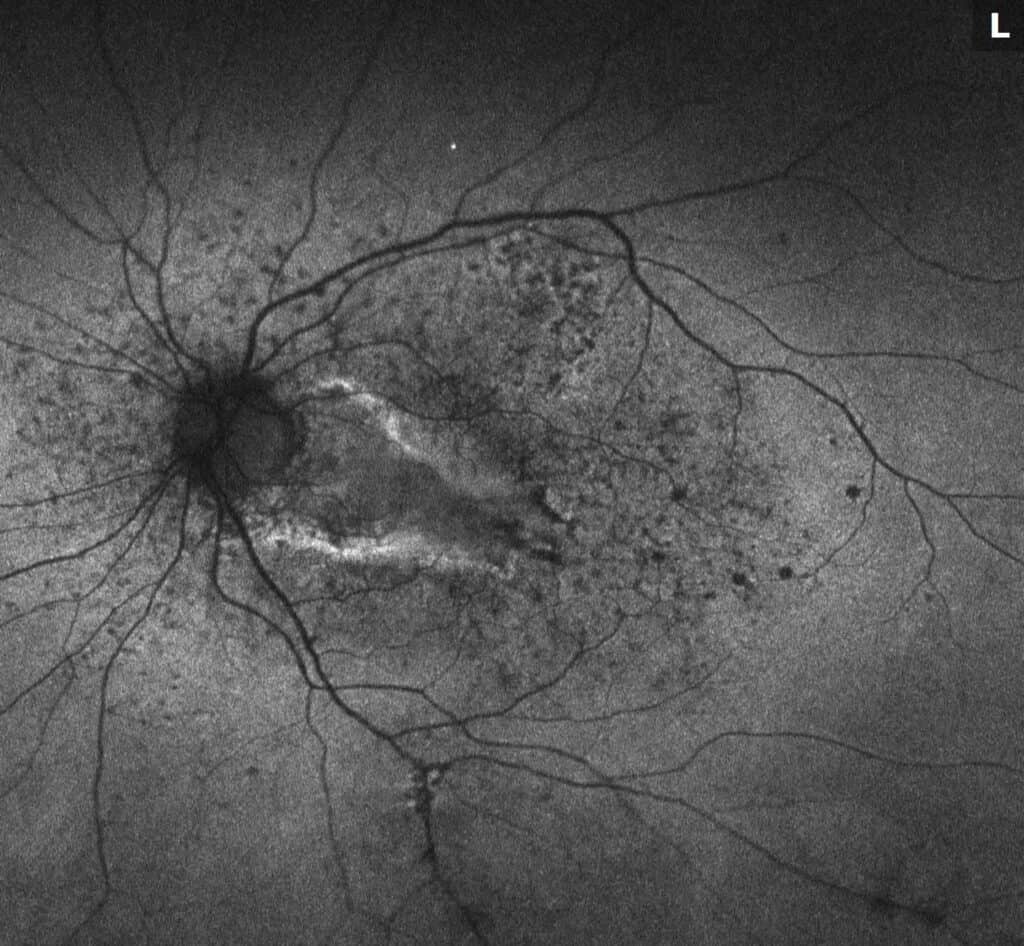
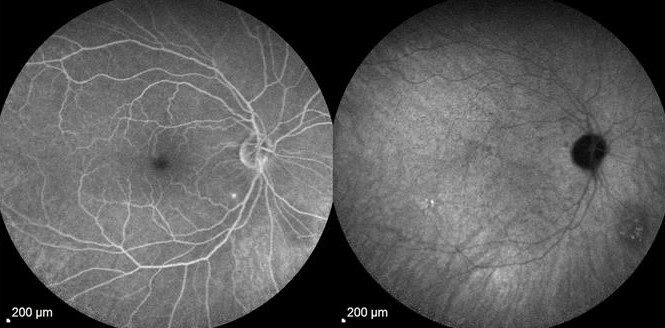
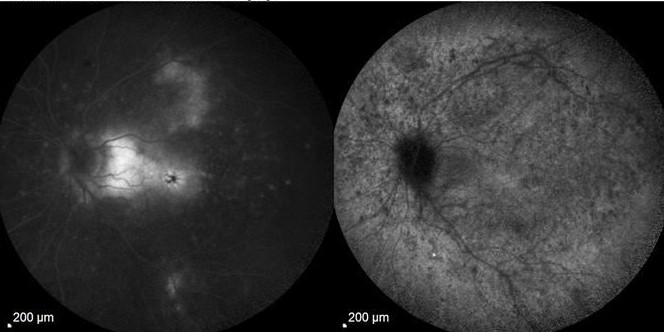
What is Epiretinal membrane (ERM)? Epiretinal membrane (ERM) is a condition characterized by the formation of avascular tissue or membrane over the retina. It often leads to vision loss or distortion, particularly when the macula is affected. The macula, a region with a high concentration of cones, is essential for color perception and fine detail.
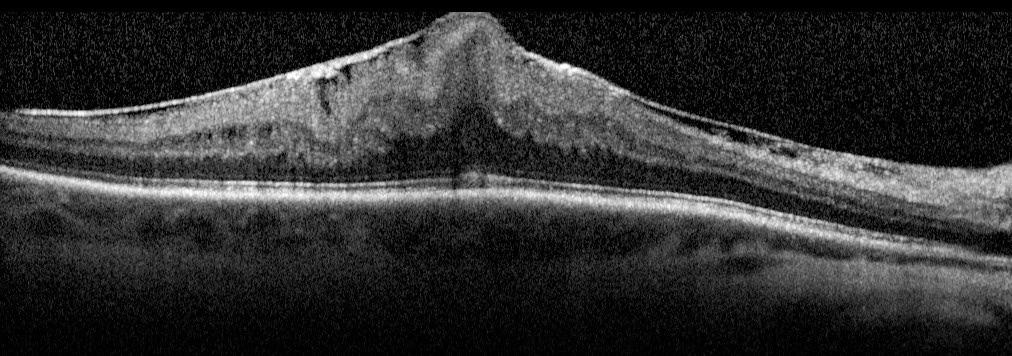
Symptoms of ERM
- Common: Distortion of straight lines (appear wavy), difficulty with fine details, and central blind spots.
- Severe: Double vision, light sensitivity, and size distortion.
These symptoms arise due to pulling and wrinkling of the macula caused by the membrane.
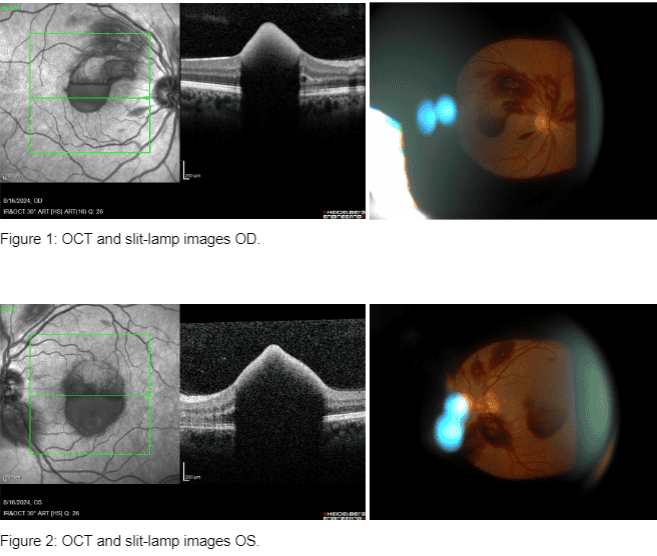
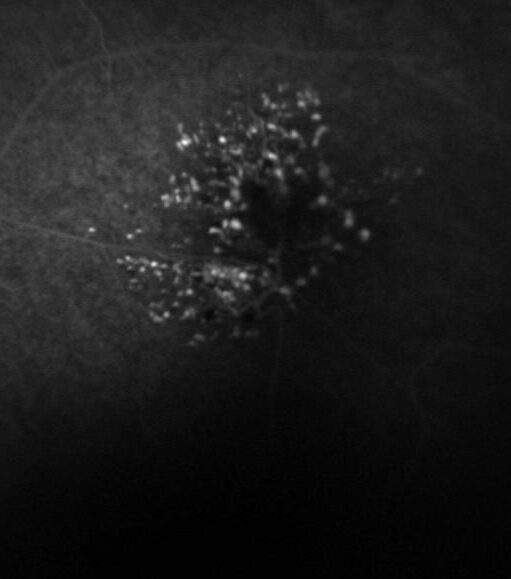
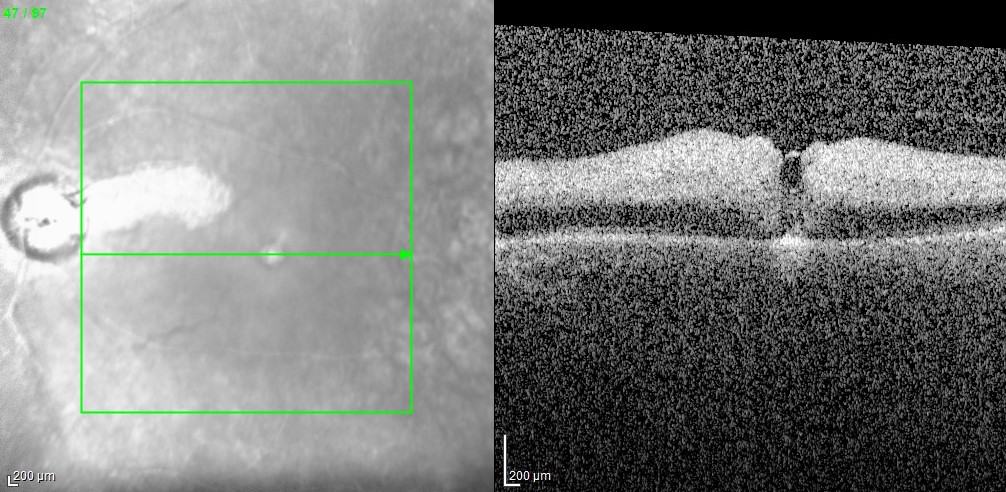
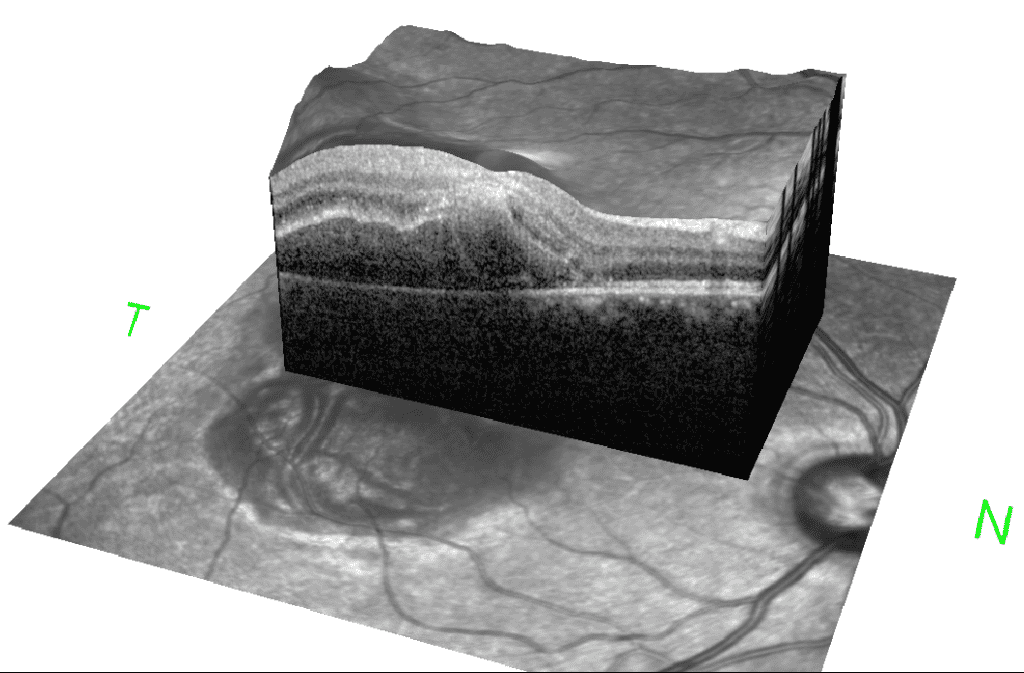
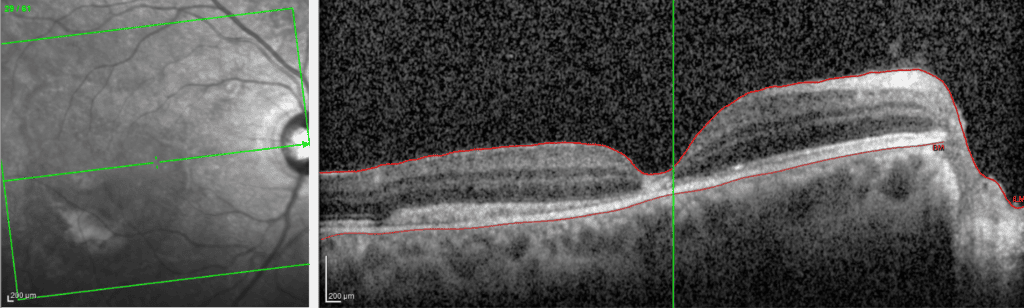
Diagnosis: ERM is typically identified during routine eye exams, utilizing:
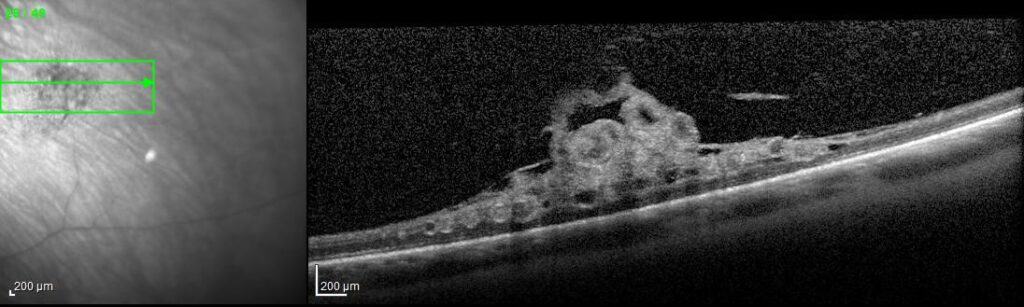
- Ocular coherence tomography (OCT)
- Fluorescein angiography (FA)
Risk Factors: Individuals are at greater risk for ERM due to factors such as:
- Age: ERM is a common condition among older adults, with studies indicating that 20% of individuals over 75 exhibit signs of the disease.
- Diabetes
- Retinal tears
- Inflammation
- Trauma to the eye
- Previous eye surgeries
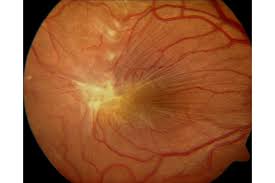
Types of ERM:
- Idiopathic ERM: Develops without pre-existing conditions.
- Secondary ERM: Associated with other conditions like posterior vitreous detachment (PVD), where the vitreous gel separates from the retina.
Treatment Options: Treatment is tailored to the patient, depending on the severity and progression of symptoms.
1. Surgical Options:
- Procedure:
- A vitrectomy involves removing the vitreous gel and replacing it with a saline solution.
- Membrane peeling is performed to remove the ERM.
- Indications: Surgery is considered when vision distortion worsens progressively and interferes with daily activities.
- Outcomes: Most patients experience gradual improvement over months. Recovery time varies based on ERM severity, duration, and other factors.
2. Non-Surgical Options: Recent studies have explored non-surgical approaches, including:
- Anti-VEGF Injections:
- Anti-vascular endothelial growth factor (anti-VEGF) injections work by reducing retinal edema and inflammation, which can alleviate symptoms and improve vision in patients with concurrent macular edema.
- These injections are particularly effective in stabilizing the condition and are often considered for patients who are not immediate candidates for surgery.
- Steroid Drops and NSAID Drops:
- Steroid drops help reduce inflammation and retinal swelling, providing symptom relief.
- NSAID drops are used to manage pain and inflammation, often as an adjunctive therapy following surgery or injections.
- These medications are typically prescribed for milder cases or as part of a broader treatment plan to delay or avoid surgery.
- Dexamethasone Implants:
- Effective in detaching ERM and improving visual outcomes.
- One study demonstrated significant improvements in patients with macular edema post-surgery (Alshahrani et al.), though repeated injections may be necessary to sustain benefits beyond six months (Chang et al.).
When to Choose Surgery vs. Non-Surgical Options: Dr. Shah recommends surgery when visual symptoms progressively worsen to the point of interfering with daily activities and quality of life, without signs of spontaneous improvement. Non-surgical options, such as anti-VEGF injections or steroid drops, are advised for patients with milder symptoms, concurrent macular edema, or those who are not yet ready for surgery. These treatments can help stabilize the condition and improve visual function.
Conclusion: While ERM can significantly impact vision, advancements in both surgical and non-surgical treatments offer effective solutions. Dr. Shah evaluates each case of ERM on an individual basis, considering the patient’s unique needs and circumstances. Depending on the severity and progression of symptoms, Dr. Shah is able to provide either surgical or non-surgical treatments to achieve the best possible outcomes. Timely diagnosis and a personalized treatment approach are essential for optimal outcomes.
Alshahrani, Saeed T et al. “Epiretinal membrane after branch retinal vein occlusion: Separation
after dexamethasone implant injection.” American Journal of Ophthalmology Case
Reports vol. 25 101252. 1 Jan. 2022, doi:10.1016/j.ajoc.2021.101252
Chang, Yo-Chen et al. “Dexamethasone Intravitreal Implant (Ozurdex) for Long-Term Macular
Edema after Epiretinal Membrane Peeling Surgery.” Journal of Ophthalmology vol. 2018
5832186. 10 Dec. 2018, doi:10.1155/2018/5832186.